Grams molecules and moles worksheet – Embark on a captivating journey into the realm of chemistry with our comprehensive Grams, Molecules, and Moles Worksheet. This meticulously crafted guide unravels the intricacies of mass, units, and their profound impact on chemical calculations, empowering you with a deep understanding of the fundamental principles that govern the world of chemistry.
Delve into the concepts of grams, molecules, and moles, unraveling their взаимосвязь and significance in quantitative chemical analysis. Prepare to master the art of converting between these units, unlocking the secrets of stoichiometry, molar mass, and molecular mass. Explore the practical applications of these concepts in diverse fields, ranging from medicine to environmental science, gaining a profound appreciation for their invaluable role in shaping our world.
Introduction
Mass is a fundamental property of matter that measures the amount of substance present. In chemistry, mass plays a crucial role in understanding the composition and behavior of chemical substances.
To quantify mass, we use units such as grams (g), kilograms (kg), and pounds (lb). The gram is the base unit of mass in the metric system, and it is defined as one-thousandth of a kilogram (1 g = 0.001 kg).
Molecules
Molecules are the smallest units of a substance that retain its chemical properties. They are composed of atoms, which are the basic building blocks of matter. Molecules can be simple, consisting of just a few atoms, or complex, containing thousands of atoms.
Moles
The mole is a unit of measurement used to quantify the amount of substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities (atoms, molecules, ions, or electrons). The mole is a convenient unit for expressing the amount of substance because it allows us to relate the mass of a substance to the number of molecules or atoms present.
Relationship between Grams, Molecules, and Moles
The relationship between grams, molecules, and moles is determined by the molar mass of the substance. The molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol).
To convert between grams and moles, we use the following formula:
Number of moles = Mass (in grams) / Molar mass
Conversely, to convert between moles and grams, we use the formula:
Mass (in grams) = Number of moles × Molar mass
Converting between Grams, Molecules, and Moles
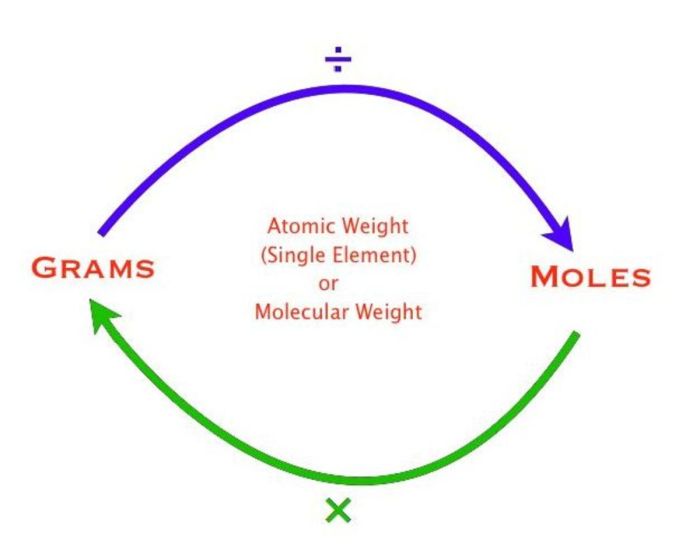
Converting between grams, molecules, and moles is essential for stoichiometric calculations. Here’s a step-by-step guide to help you master these conversions:
Grams to Molecules, Grams molecules and moles worksheet
To convert grams to molecules, follow these steps:
- Determine the molar mass of the substance using the periodic table.
- Divide the given mass in grams by the molar mass to obtain the number of moles.
- Multiply the number of moles by Avogadro’s number (6.022 x 10 23molecules/mol) to get the number of molecules.
Example:
Convert 25 grams of sodium chloride (NaCl) to molecules.
- Molar mass of NaCl = 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
- Moles of NaCl = 25 g / 58.44 g/mol = 0.428 mol
- Number of molecules = 0.428 mol x 6.022 x 10 23molecules/mol = 2.58 x 10 23molecules
Molecules to Grams
To convert molecules to grams, follow these steps:
- Divide the given number of molecules by Avogadro’s number to obtain the number of moles.
- Multiply the number of moles by the molar mass of the substance to get the mass in grams.
Example:
Convert 5.0 x 10 24molecules of carbon dioxide (CO 2) to grams.
- Molar mass of CO 2= 12.01 g/mol + 2 x 16.00 g/mol = 44.01 g/mol
- Moles of CO 2= 5.0 x 10 24molecules / 6.022 x 10 23molecules/mol = 8.31 mol
- Mass of CO 2= 8.31 mol x 44.01 g/mol = 364.4 g
Grams to Moles
To convert grams to moles, simply divide the given mass in grams by the molar mass of the substance.
Example:
Convert 150 grams of glucose (C 6H 12O 6) to moles.
- Molar mass of C 6H 12O 6= 180.16 g/mol
- Moles of glucose = 150 g / 180.16 g/mol = 0.832 mol
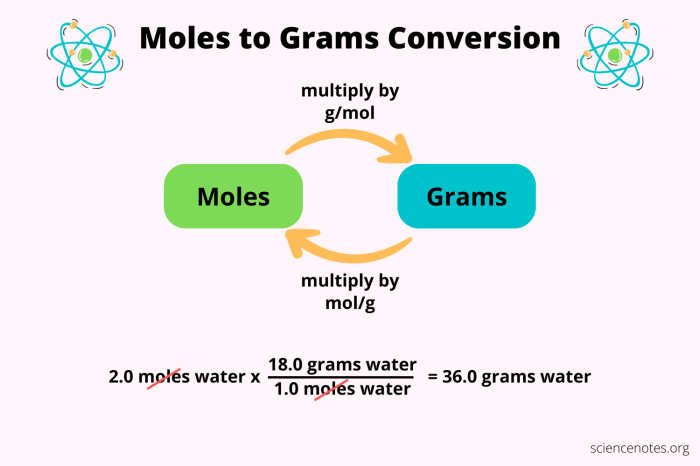
Moles to Grams
To convert moles to grams, multiply the given number of moles by the molar mass of the substance.
Example:
Convert 2.5 moles of magnesium sulfate (MgSO 4) to grams.
- Molar mass of MgSO 4= 24.31 g/mol + 32.07 g/mol + 4 x 16.00 g/mol = 120.37 g/mol
- Mass of MgSO 4= 2.5 mol x 120.37 g/mol = 300.9 g
Practice Problems:
- Convert 40 grams of water (H 2O) to molecules.
- Convert 3.5 x 10 25molecules of ammonia (NH 3) to grams.
- Convert 125 moles of sodium chloride (NaCl) to grams.
- Convert 1.8 kilograms of glucose (C 6H 12O 6) to moles.
Molar Mass and Molecular Mass: Grams Molecules And Moles Worksheet
Molar mass and molecular mass are two important concepts in chemistry. Molar mass is the mass of one mole of a substance, while molecular mass is the mass of one molecule of a substance.
To calculate the molar mass of a substance, you need to know the atomic masses of the elements that make up the substance. The atomic mass of an element is the average mass of all the isotopes of that element.
Once you know the atomic masses of the elements, you can calculate the molar mass of the substance by adding up the atomic masses of all the atoms in the substance.
To calculate the molecular mass of a substance, you need to know the number of atoms of each element in the molecule. Once you know the number of atoms of each element, you can calculate the molecular mass of the substance by adding up the atomic masses of all the atoms in the molecule.
Example
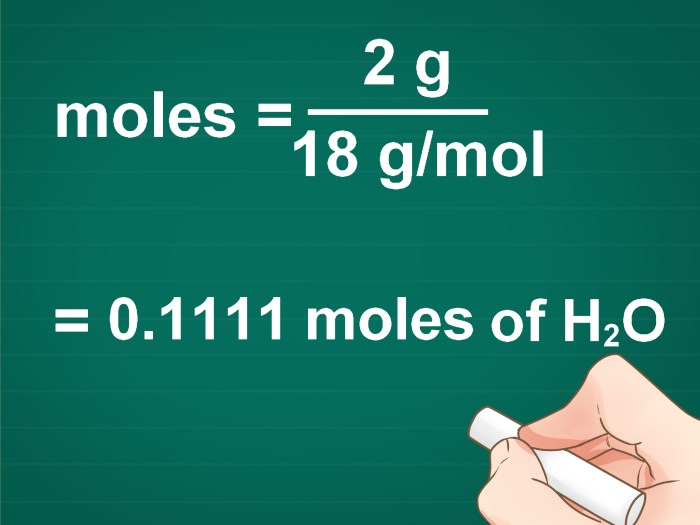
What is the molar mass of water (H 2O)?
The atomic mass of hydrogen is 1.008 amu, and the atomic mass of oxygen is 15.9994 amu. Therefore, the molar mass of water is 1.008 amu + 1.008 amu + 15.9994 amu = 18.01534 amu.
Practice Problem
What is the molecular mass of carbon dioxide (CO 2)?
Stoichiometry
Stoichiometry is the branch of chemistry that deals with the quantitative relationships between reactants and products in chemical reactions. It helps us predict the amount of reactants and products involved in a reaction, as well as the mole ratios between them.
Stoichiometry is essential for various applications, including:
- Predicting the amount of reactants and products needed or produced in a reaction
- Balancing chemical equations
- Determining the limiting reactant in a reaction
- Calculating the theoretical yield of a reaction
- Understanding the stoichiometric coefficients in a balanced chemical equation
Stoichiometry relies heavily on balanced chemical equations, which provide the mole ratios of reactants and products. By analyzing the coefficients in a balanced equation, we can determine the number of moles of each substance involved in the reaction.
For example, consider the following balanced chemical equation:
H2+ O 2→ 2H 2O
This equation tells us that 2 moles of hydrogen (H 2) react with 1 mole of oxygen (O 2) to produce 2 moles of water (H 2O). The mole ratios can be expressed as:
- 2 moles H 2: 1 mole O 2
- 2 moles H 2: 2 moles H 2O
- 1 mole O 2: 2 moles H 2O
These mole ratios are crucial for stoichiometric calculations and predicting the quantities of reactants and products involved in the reaction.
Percent Composition
Percent composition refers to the relative amount of each element present in a compound, expressed as a percentage. It provides insights into the elemental makeup of a substance and is crucial for understanding its properties and reactivity.
To calculate the percent composition of a compound, follow these steps:
- Determine the molar mass of the compound by adding the atomic masses of all its constituent atoms.
- Calculate the mass of each element in the compound by multiplying its atomic mass by the number of atoms of that element present in the compound’s formula.
- Divide the mass of each element by the molar mass of the compound and multiply by 100 to obtain the percent composition.
For example, consider carbon dioxide (CO 2). The molar mass of CO 2is 44 g/mol (12 g/mol for carbon + 2 x 16 g/mol for oxygen). The mass of carbon in CO 2is 12 g/mol, and the mass of oxygen is 32 g/mol.
Therefore, the percent composition of CO 2is:
- Percent composition of carbon = (12 g/mol / 44 g/mol) x 100 = 27.27%
- Percent composition of oxygen = (32 g/mol / 44 g/mol) x 100 = 72.73%
Empirical and Molecular Formulas
Chemical formulas represent the elemental composition of a compound. Empirical formulas show the simplest whole-number ratio of elements in a compound, while molecular formulas represent the actual number of atoms of each element in a molecule of the compound.
Determining Empirical Formulas
To determine the empirical formula of a compound, we need to know the mass of each element present in the compound. We can then divide the mass of each element by its atomic mass to get the number of moles of each element.
The mole ratio of the elements is then simplified to the smallest whole-number ratio, which gives us the empirical formula.
Determining Molecular Formulas
To determine the molecular formula of a compound, we need to know the empirical formula and the molar mass of the compound. The molar mass is the mass of one mole of the compound, and it can be determined experimentally.
Once we have the molar mass and the empirical formula, we can divide the molar mass by the empirical formula mass to get the molecular formula.
Example
Consider a compound with a mass of 10.0 g that contains 4.0 g of carbon, 1.0 g of hydrogen, and 5.0 g of oxygen. The empirical formula of this compound is CH 2O, and its molar mass is 30.0 g/mol.
Therefore, the molecular formula of the compound is C 2H 4O 2.
Practice Problems
- Determine the empirical formula of a compound that contains 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen.
- Determine the molecular formula of a compound with an empirical formula of CH2O and a molar mass of 60.0 g/mol.
Applications of Grams, Molecules, and Moles
Grams, molecules, and moles are fundamental units in chemistry that play crucial roles in various scientific fields. Their applications extend beyond the laboratory, finding practical uses in medicine, engineering, and environmental science.
In medicine, the precise measurement of grams and moles is essential for drug preparation and dosage calculations. By accurately determining the amount of a drug substance present in a given mass, healthcare professionals can ensure accurate and effective treatment for patients.
In Engineering
In engineering, the knowledge of grams, molecules, and moles is crucial for material science and chemical processes. For instance, in the production of alloys, engineers need to carefully control the proportions of different elements to achieve the desired properties. By measuring the mass and moles of each element, they can precisely adjust the composition of the alloy to meet specific performance requirements.
In Environmental Science
In environmental science, grams, molecules, and moles are essential for monitoring and controlling pollution. By measuring the concentration of pollutants in air, water, and soil, scientists can assess the environmental impact and develop strategies to mitigate their effects. For example, measuring the number of moles of carbon dioxide emitted from industrial processes helps in regulating greenhouse gas emissions and combating climate change.
Questions Often Asked
What is the difference between grams and moles?
Grams measure the mass of a substance, while moles measure the amount of substance present. One mole of a substance contains 6.022 x 10^23 particles (atoms, molecules, or ions) of that substance.
How do I convert between grams and moles?
To convert grams to moles, divide the mass in grams by the molar mass of the substance. To convert moles to grams, multiply the number of moles by the molar mass.
What is stoichiometry?
Stoichiometry is the study of the quantitative relationships between reactants and products in chemical reactions. It allows us to predict the amount of reactants and products involved in a given reaction.